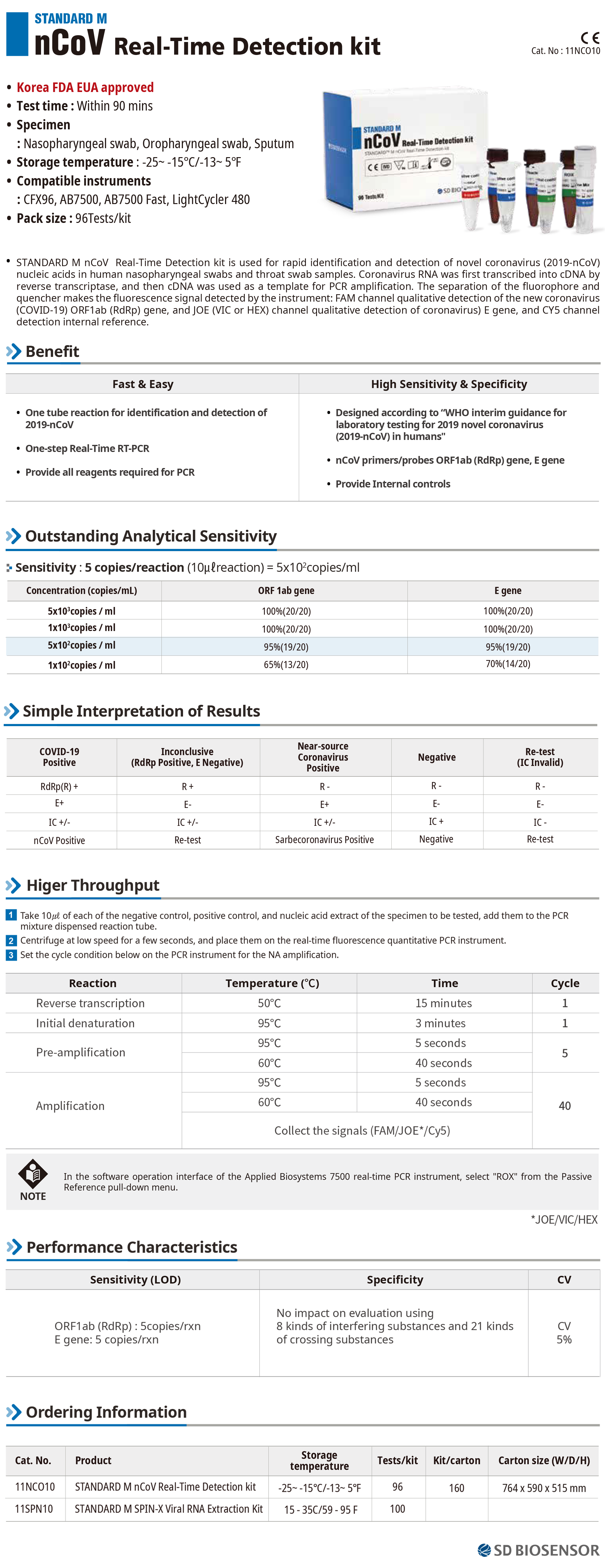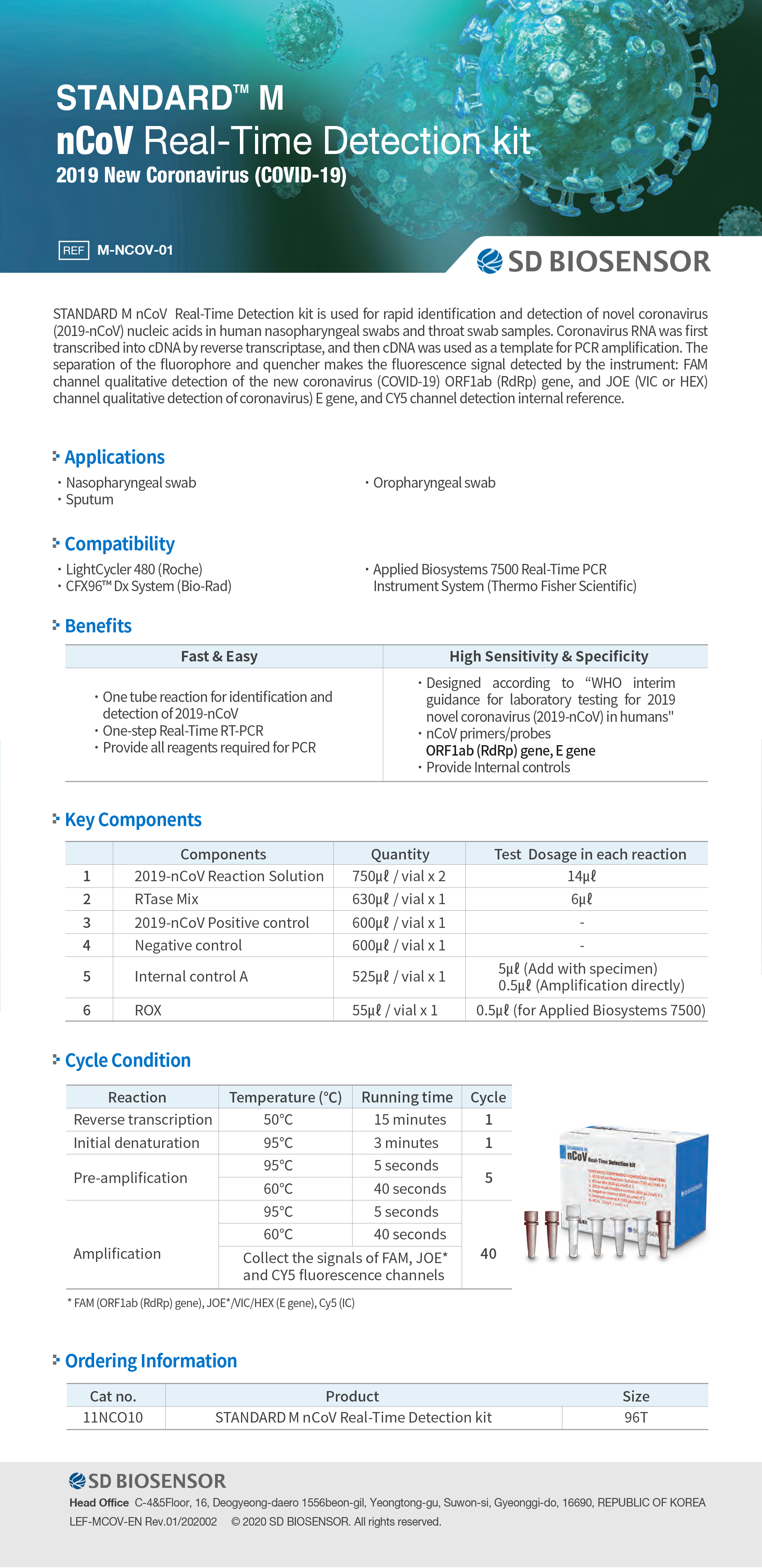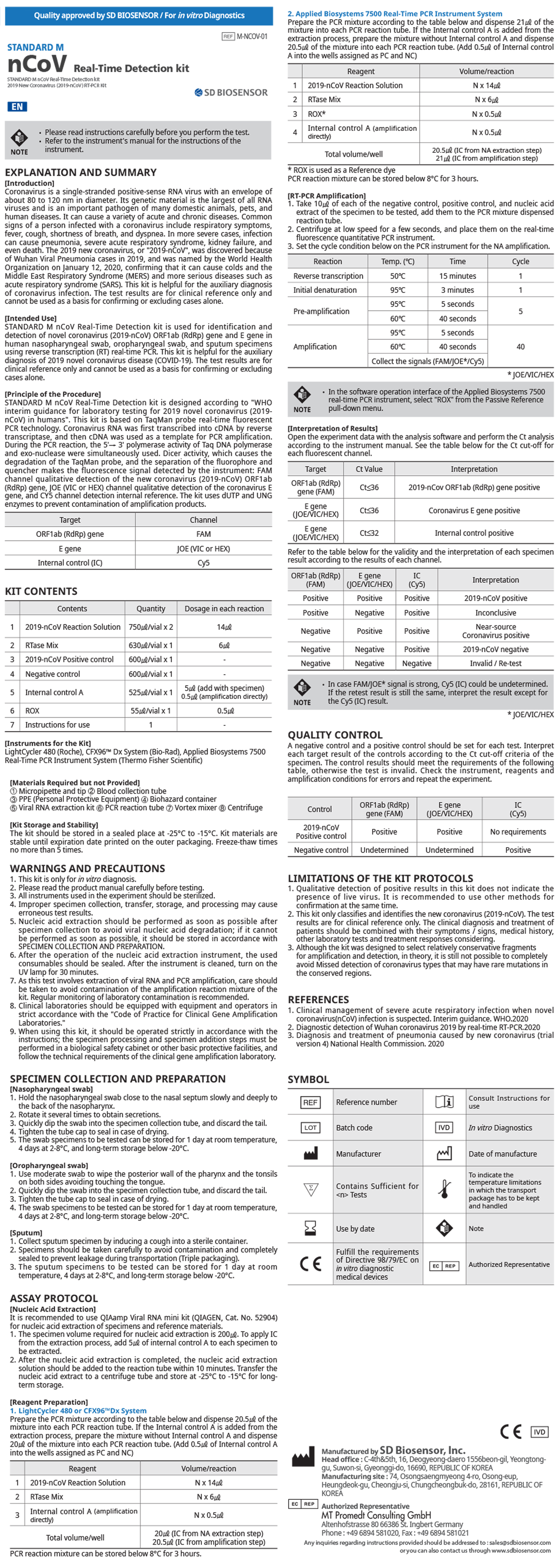
nCoV Real-Time Detection kit
CE-IVD, Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA)
On January 11, 2020, Chinese health authorities preliminarily identified more than 40 human infections with a novel coronavirus in an outbreak of pneumonia under investigation in Wuhan City, Hubei Province, China. The Chinese authorities identified a new type of coronavirus (novel coronavirus, named as COVID-19), which was isolated on 7 January 2020. nCoV Real-Time Detection kit is the One-Step Reverse Transcription Real-Time PCR Kit designed to detect Novel Corona virus (COVID-19) qualitatively through Reverse Transcription reaction and Real-Time Polymerase Chain Reaction.
nCoV Real-Time Detection kit is CE approved and KFDA approved product. It has been widely used in Korea and several other countries during this pandemic. It is registered with the US FDA and have received the EUA. The CDC has purchased initial 300,000 kits.
For ordering this test kit, please send your enquiry to Justin Nguyen, President & COO of Genosolutions, Inc. Justin@genosolutions.net ; Direct +1-858-733-2368.
nCoV Real-Time Detection kit Description